- Elisabeth A. George,
- Leslie Castelo-Soccio,
- Elana Putterman,
- Helena Kuhn,
- Carlos Wambier,
- Abrar Qureshi &
- Eunyoung Cho
Scientific Reports volume 11, Article number: 21034 (2021) Cite this article
- 2359 Accesses
- 4 Citations
- 4 Altmetric
- Metrics details
Abstract
Patients with alopecia areata (AA) may experience episodic disease flares characterized by increasing hair loss that follow a seasonal pattern. However, no studies have examined whether specific climate factors contribute to the seasonal pattern of AA flares. Using Spearman rank correlation analyses, we assessed the association between climate variables and AA flare frequency per month in 336 children with AA in Philadelphia, Pennsylvania. Region-specific monthly values for average ambient temperature, air pressure, cloudiness, hours of sunlight, relative humidity, number of days with sun, number of days with rain, volume of precipitation, wind gust, wind speed, and UV index from January 2015 to December 2017 were obtained from World Weather Online. We found significant (P < 0.05) correlations between AA flare frequency and UV index (R = − 0.66), precipitation (R = − 0.66), number of days with rain (R = − 0.70), number of days with sun (R = 0.62), and air pressure (R = 0.80). Stratified analyses showed even stronger associations with UV index and precipitation in patients with an atopic comorbidity. New significant correlations appeared with temperature, wind speed, and UV index of the prior month. However, in patients who did not have atopic comorbidities, we generally observed weaker and non-significant correlations between climate and AA flare frequency. This study suggests that certain climate factors may mediate the seasonal pattern of AA flares and may contribute to AA pathogenesis. Atopic AA patients may be more susceptible to the influence of climate compared to those with no history of atopy.
Introduction
We previously found that pediatric alopecia areata (AA) flares follow a seasonal pattern, with a greater proportion of flares occurring in the late fall and a smaller proportion towards the end of spring1. Stratified analyses based on the presence of an atopic comorbidity suggest that the clinical course of AA differs between atopic patients and their counterparts1. We found that the greatest proportion of flares occurred during the winter in atopic AA patients. However, the greatest proportion of flares occurred during the fall in patients without atopy.
Whether climate factors contribute to the seasonal pattern of pediatric AA flares remains unknown. In the present study, we hypothesize that regional UV index, a standardized measure of the strength of sunburn-producing UV radiation, may partly contribute to this pattern, since UV-B irradiation of skin triggers vitamin D synthesis2,3. Meta-analyses suggest that serum levels of vitamin D are lower in patients with AA, supporting the potential role of vitamin D in the pathogenesis of AA2. However, only one included study was based on AA patients in the United States (US) suggesting lack of data in the US. Using UV index as an indirect surrogate of serum vitamin D levels, in the present study, we explore the association between AA flare frequency and various climate factors, including UV index.
Methods
Medical charts of 457 children in Philadelphia, Pennsylvania with AA, alopecia totalis (AT) or alopecia universalis (AU) were reviewed. Of these, 336 children with dates of AA flare episodes in 2017 were included. Included episodes were the onset of hair loss, the first flare, and the second flare. Flares were defined as an increase in scalp hair loss noted by either patient or caregiver. Increased hair loss occurring immediately after a course of oral steroids was not included as a true flare episode to limit the confounding effect of steroids. Data from 518 episodes of flares were collected to calculate the frequency of flares during each month. The monthly values of climate variables in Philadelphia during 2015 to 2017 were obtained from World Weather Online, including average ambient temperature, UV index, air pressure, relative humidity, cloudiness, wind gust, wind speed, number of days with rain, volume of precipitation, number of days with sun, and hours of sunlight. Spearman rank correlation analyses were conducted using STATA version 15 to assess the correlations between these climate variables and AA flare frequency per month. To explore lag-time effects, UV index of the prior month was also analyzed.
Results
Demographic information of the subjects was previously reported1. The male-to-female ratio was 1:1.5. The majority of our subjects self-identified as White (52.2%), followed by Black (19.6%), Hispanic or Latino (8.9%), Asian (4.1%), Indian (3.9%), Other (8.9%), and unknown (< 3%). The majority of our subjects had a documented atopic comorbidity (63.8%), with 87 cases of atopic dermatitis (AD), 47 allergic rhinitis, 35 asthma, 14 anaphylactic food allergy, and 2 eosinophilic esophagitis.
There were significant (P < 0.05) correlations between flare frequency and UV index (R = − 0.66), precipitation (R = − 0.66), number of days with rain (R = − 0.70), number of days with sun (R = 0.62), and air pressure (R = 0.80). (Table 1 and Fig. 1). Stratified analyses based on the presence of an atopic comorbidity revealed that the correlations between AA flares and certain climate variables, namely UV index and precipitation, were stronger and significant only in atopic patients with AA (Table 1). In atopic AA patients, new significant correlations appeared with temperature (R = − 0.75), wind speed (R = 0.64), and UV index of the prior month (R = − 0.61). However, in patients who did not have atopic comorbidities, we generally observed weaker and non-significant correlations between climate and AA flare frequency. Out of the twelve examined climate variables, only three remained significant in patients without atopic comorbidities: number of days with rain (R = − 0.63), number of days with sun (R = 0.58), and air pressure (R = 0.59).Table 1 Correlation coefficients (Rs) between the frequency of alopecia areata (AA) flares and meteorological variables per month in Philadelphia, Pennsylvania, for children with AA, for children with comorbid AA and atopic conditions, and for children with AA without atopic conditions.
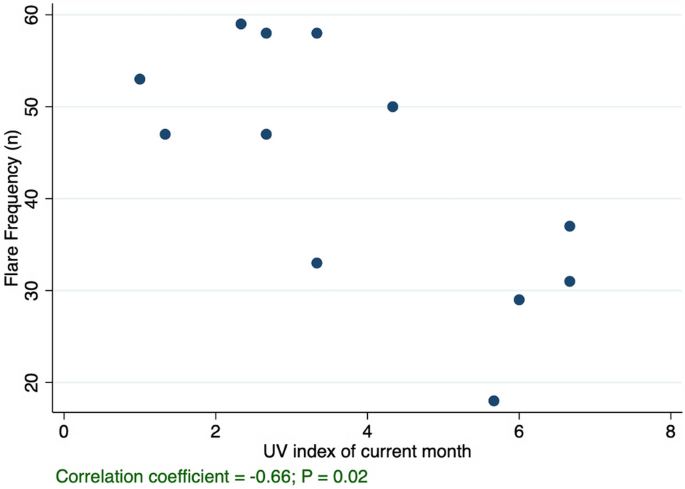
Discussion
As predicted, we found a strong inverse correlation between UV index and AA flare frequency, which may partly explain the seasonal pattern of AA that we previously observed1. Furthermore, this correlation was stronger among atopic AA patients compared with patients without atopy.
Our findings regarding UV index may support the potential role of vitamin D in AA pathogenesis as vitamin D deficiency is more common in the fall and winter months when UV exposure is lower2,4. UV-induced immunosuppression may contribute to these findings as well5. We also found significant correlations between flare frequency and precipitation, number of days with rain, and air pressure. Since these three climate factors correlated with UV index during the 3-year period (R = − 0.80 to 0.65), UV index may partly account for their significant association with AA flare frequency. While UV index was strongly correlated with temperature (R = 0.92) and UV index of the prior month (R = 0.82), associations between these climate variables and flare frequency were only significant in atopic AA patients.
Stratified analyses based on comorbid atopy suggest that atopic AA patients may be more susceptible to the influence of climate compared to those with no history of atopy (Table 1). This may be related to the fact that atopic conditions are associated with climate. Prior studies have found convincing evidence that atopic conditions have seasonal patterns and may be influenced by climate6,7,8,9. Several population studies based in the US10, Turkey11, Denmark8, and Norway12, but not others7,13, have suggested that there is an inverse relationship between temperature and AD. Lower temperatures correlated with increased AD prevalence10, more office visits8,11,12, more hospitalizations8, and more AD prescription medication utilization8. Prior studies have also found that some climate factors such as precipitation10, air pressure11,14, and UV index15,16,17, which were also the factors associated with AA in our study, were associated with AD. A meta-analysis based on nine individual studies found that AD was significantly associated with birth during the fall and winter seasons, compared with birth during spring18. One theory to explain the effects of season of birth on atopic conditions involves vitamin D produced by skin in response to UV-B radiation19. A recent systematic review supported the role of vitamin D in the pathogenesis of AD20.
Our study was based in a geographic region with four distinct seasons, which provides a wide range of climate variation. Our focus on a pediatric population may be advantageous, as high-dose supplemental vitamin D intake is less prevalent among children compared to adults21. This would allow for more robust influence of UV exposure on flare frequency, if serum vitamin D levels truly mediate the correlation between UV index and AA flare frequency. However, we were not able to explore the relationship between atopic severity and that of AA due to lack of information on atopic severity, which is one limitation of this study. Previous research indicates that allergy may contribute to AA pathogenesis in a subset of patients22. In fact, atopic AA patients may benefit from antihistamines as adjunct therapy to support hair regrowth23,24. Thus, it is reasonable to wonder whether antihistamine use and atopy severity contribute to the seasonal AA flare pattern that is observed in atopic AA patients. Future research studies including multivariable analyses may be useful to further define the relationship between climate, atopic flares, and those of AA.
In conclusion, UV exposure and other climate factors may be partially responsible for the seasonal pattern of AA flares and may contribute to AA pathogenesis. Physicians may counsel pediatric patients and their caregivers on these relationships between climate and AA flare frequency. A useful teaching analogy may be to think of the hair as leaves, with increased shedding during fall and more growth during spring. The seasonal patterns of AA flares should be documented in adults and pediatric populations in various geographic regions to better understand of the mechanisms.
Data availability
Dr. Cho and Ms. George had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Putterman, E. & Castelo-Soccio, L. Seasonal patterns in alopecia areata, totalis, and universalis. J. Am. Acad. Dermatol. 79(5), 974–975 (2018).
- Lee, S., Kim, B. J., Lee, C. H. & Lee, W. S. Increased prevalence of vitamin D deficiency in patients with alopecia areata: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 32(7), 1214–1221 (2018).
- Abhimanyu, A. & Coussens, A. K. The role of UV radiation and vitamin D in the seasonality and outcomes of infectious disease. Photochem. Photobiol. 16(3), 314–338 (2017).
- Orces, C., Lorenzo, C. & Guarneros, J. E. The prevalence and determinants of vitamin D Inadequacy among U.S. older adults: National health and nutrition examination survey 2007–2014. Cureus. 11(8), e5300 (2019).
- González Maglio, D. H., Paz, M. L. & Leoni, J. Sunlight effects on immune system: Is there something else in addition to UV-induced immunosuppression?. Biomed. Res. Int. 2016, 1934518 (2016).
- Hwang, C. Y. et al. Prevalence of atopic dermatitis, allergic rhinitis and asthma in Taiwan: A national study 2000 to 2007. Acta Derm. Venereol. 90(6), 589–594 (2010).
- Fleischer, A. B. Jr. Atopic dermatitis: The relationship to temperature and seasonality in the United States. Int. J. Dermatol. 58(4), 465–471 (2019).
- Hamann, C. R. et al. The effects of season and weather on healthcare utilization among patients with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 32(10), 1745–1753 (2018).
- Dunlop, J. H., Keller, J. P., Peng, R. D. & Keet, C. A. The effect of season of birth on atopic dermatitis and food allergy. Ann. Allergy Asthma Immunol. 125(2), 221-223.e222 (2020).
- Silverberg, J. I., Hanifin, J. & Simpson, E. L. Climatic factors are associated with childhood eczema prevalence in the United States. J. Invest. Dermatol. 133(7), 1752–1759 (2013).
- Karagün, E., Yıldız, P. & Cangür, Ş. Effects of climate and air pollution factors on outpatient visits for eczema: A time series analysis. Arch. Dermatol. Res. 313(1), 49–55 (2021).
- Mohn, C. H. et al. Incidence trends of atopic dermatitis in infancy and early childhood in a nationwide prescription registry study in Norway. JAMA Netw. Open. 1(7), e184145 (2018).
- Narla, S., Hsu, D. Y., Thyssen, J. P. & Silverberg, J. I. Inpatient financial burden of atopic dermatitis in the United States. J. Invest. Dermatol. 137(7), 1461–1467 (2017).
- Li, A., Fan, L., Xie, L., Ren, Y. & Li, L. Associations between air pollution, climate factors and outpatient visits for eczema in West China Hospital, Chengdu, south-western China: A time series analysis. J. Eur. Acad. Dermatol Venereol. 32(3), 486–494 (2018).
- Silverberg, J., Hanifin, J., Simpson, E. Relative humidity, dew point, indoor heating and ultraviolet index are associated with the prevalence of eczema. J. Investig. Dermatol. (2012).
- Fuertes, E., Flohr, C., Silverberg, J. I., Standl, M. & Strachan, D. P. Global associations between UVR exposure and current Eczema prevalence in children from ISAAC phase three. J. Invest. Dermatol. 137(6), 1248–1256 (2017).
- Stefanovic, N., Irvine, A. D. & Flohr, C. The role of the environment and exposome in atopic dermatitis. Curr. Treat. Options Allergy. 2021, 1–20 (2021).
- Calov, M. et al. The association between season of birth and atopic dermatitis in the Northern Hemisphere: A systematic review and meta-analysis. J. Allergy Clin. Immunol. Pract. 8(2), 674-680.e675 (2020).
- Matsui, T. et al. Food allergy is linked to season of birth, sun exposure, and vitamin D deficiency. Allergol. Int. 68(2), 172–177 (2019).
- Huang, C. M., Lara-Corrales, I. & Pope, E. Effects of Vitamin D levels and supplementation on atopic dermatitis: A systematic review. Pediatr Dermatol. 35(6), 754–760 (2018).
- Bailey, R. L. et al. Estimation of total usual calcium and vitamin D intakes in the United States. J. Nutr. 140(4), 817–822 (2010).
- Zhang, X. & McElwee, K. J. Allergy promotes alopecia areata in a subset of patients. Exp. Dermatol. 29(3), 239–242 (2020).
- Inui, S., Nakajima, T., Toda, N. & Itami, S. Fexofenadine hydrochloride enhances the efficacy of contact immunotherapy for extensive alopecia areata: Retrospective analysis of 121 cases. J. Dermatol. 36(6), 323–327 (2009).
- Ito, T., Fujiyama, T., Hashizume, H. & Tokura, Y. Antihistaminic drug olopatadine downmodulates T cell chemotaxis toward CXCL10 by reducing CXCR3 expression, F-actin polymerization and calcium influx in patients with alopecia areata. J. Dermatol. Sci. 72(1), 68–71 (2013).
Author information
Authors and Affiliations
- Warren Alpert Medical School of Brown University, Providence, RI, USAElisabeth A. George
- Division of Pediatrics, Section of Dermatology, The Children’s Hospital of Philadelphia, Philadelphia, PA, USALeslie Castelo-Soccio & Elana Putterman
- University of Pennsylvania Perelman School of Medicine, The University of Pennsylvania, Philadelphia, USALeslie Castelo-Soccio & Elana Putterman
- Department of Dermatology, Warren Alpert Medical School of Brown University, 339 Eddy Street, Providence, RI, 02903, USAHelena Kuhn, Carlos Wambier, Abrar Qureshi & Eunyoung Cho
Contributions
Design and conduction of the study: E.G., A.Q., E.C. Collection, management, analysis and interpretation of Data: E.G., L.C.-S., E.P., H.K., C.W., A.Q., E.C. Preparation, review, or approval of the manuscript: E.G., L.C.-S., E.P., H.K., C.W., A.Q., E.C. Decision to submit the manuscript for publication: E.G., L.C.-S., E.P., H.K., C.W., A.Q., E.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
